



Research
Research
がん(悪性腫瘍)は、偶発的に起こる遺伝子変異の蓄積によって、異常な増殖能や不死性、他の組織へ移動する浸潤性といったいくつかの特性を細胞が獲得して生じるものと考えられており、このようながんの原因となる遺伝子の変異については既に数多くのものが知られています。その一方で、私たちの体の中には、このようながん原性の変異を抱えていても実際にはがんにならない細胞も多数存在しており、その数も種類も年を経るごとに増加していくことが分かっています。
では、変異を抱えた細胞が悪性腫瘍へと進化する発がんのきっかけは何だろうか?偶発的な変異の蓄積だけではなく、発がんのきっかけとなる何か必然的なルールがあるのではないだろうか?
私達の研究室はこのような考えをもとに、「生体組織内におけるがん細胞進化のプロセスにおいて、偶然性に支配されない必然的な事象」を探求しています。
Cancer is thought to arise from the accumulation of coincidental genetic mutations that causes cells to acquire several characteristics, such as uncontrolled proliferation, immortality, and invasiveness. There are many oncogenic gene mutations that have been identified in previous studies. It has also been shown that, however, there is a number of cells carrying such oncogenic mutations in our body that do not actually show carcinogenesis, and that the number and types of such mutant cells increase over the years.
Then, what is the trigger for carcinogenesis by which those cells harboring oncogenic mutations evolve into malignant tumors? Are there inevitabilities that trigger carcinogenesis, rather than just an accidental accumulation of mutations?
Based on this idea, our laboratory is exploring "inevitabilities that are not governed by chance in the process of cancer cell evolution in living tissues".
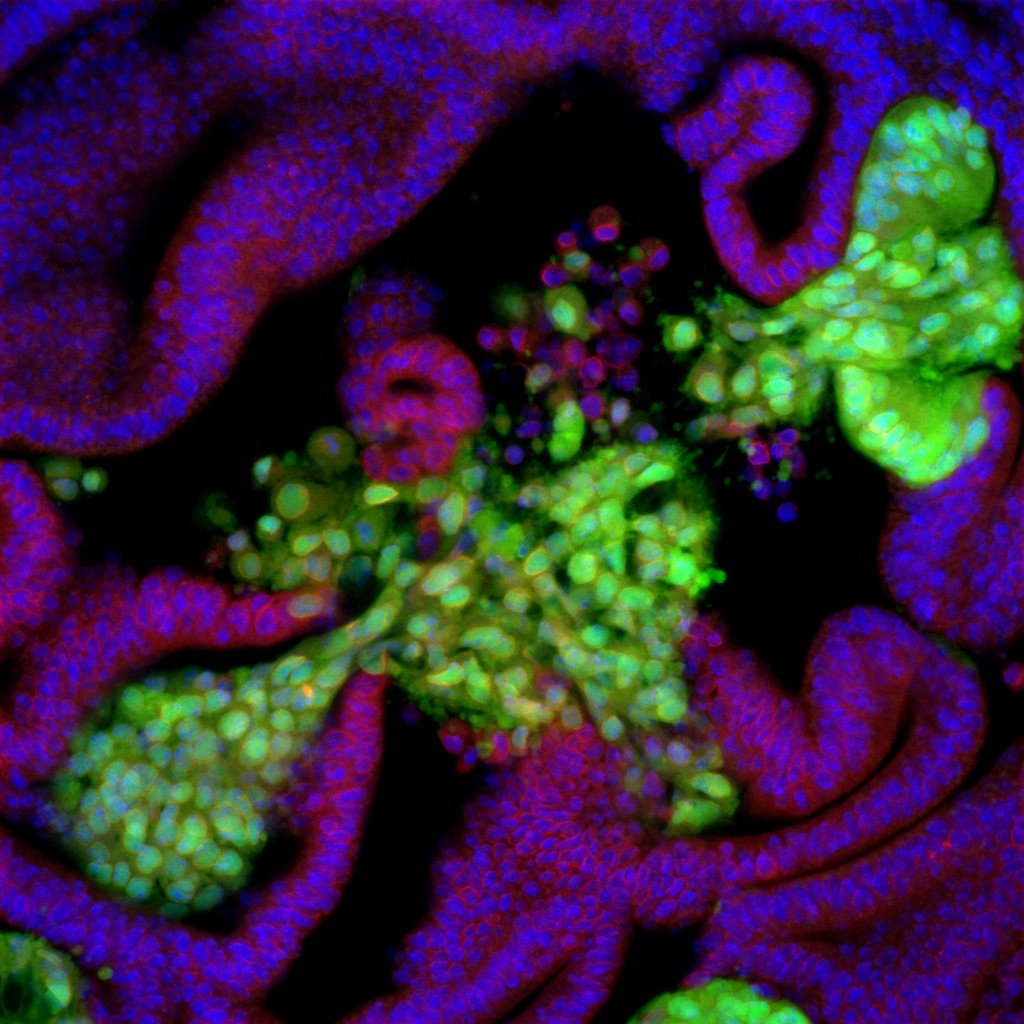
現在は主にショウジョウバエを実験モデルとして、組織に内在するがん浸潤ニッチ(Invasion Hotspots)と、腫瘍組織内の多倍体がん細胞(Polyploid Cancer Cells)の動態、そしてゲノム倍数性の変動とがん細胞進化の関係について研究を進めています。
Using Drosophila melanogaster (Fruit fly) as an experimental model, we are currently studying the tissue-intrinsic tumor invasion niche (Invasion Hotspots), Polyploid Cancer Cells, and the relationship between ploidy dynamics and cancer cell evolution.
これまでに、ショウジョウバエの遺伝学実験システムを用いて、幼虫の上皮組織(翅原基)にがん原性の変異細胞を誘導し、その動態を追跡することにより、以下のような現象を発見しました。
がん原性変異細胞が形成する腫瘍は、非常に高い確率で上皮組織内の特定の場所(浸潤ホットスポット)から基底膜を破り間質への侵入を始める。
浸潤ホットスポット以外の場所に出現したがん原性変異細胞は、同じ遺伝子変異を持っているにも関わらず、基底膜を破らず管腔側で良性腫瘍を形成する。
浸潤を始めた腫瘍細胞群のなかには、核内倍加(endoreplication)によりゲノムの多倍体化を起こしているものが含まれる。
*endoreplication…細胞周期の分裂期(M期)をスキップすることにより、S期のDNA複製を経ても分裂は起こらず、ゲノムの多倍体化・細胞サイズの巨大化を生じる細胞周期。
*多倍体化…ゲノム倍数性が、通常の2倍体から逸脱して4倍体以上の細胞(4, 8, 16, 32倍体,…)になること。
これらの現象に着目し、浸潤ホットスポットの条件、核内倍加による多倍体化のきっかけ、そして多倍体化とがん浸潤の機能的関係について調べています。また、同様の現象が他の実験モデル(マウス、哺乳類培養細胞)、さらには実際のヒトの病理組織で見られるのかどうかについても研究を進めています。
We discovered the following phenotypes of oncogenic mutant cells in the Drosophila larval tissues (wing imaginal epithelia) using genetically mosaic techniques.
Oncogenic mutant cells formed tumors that break through the underlying basement membrane and begin to invade the extracellular matrix from several specific spots (invasion hotspots) in the epithelial tissue with a very high probability.
Oncogenic mutant cells that are induced outside of the invasion hotspots do not break through the basement membrane and grow into benign tumors on the luminal side, even though they carry the same genetic mutations.
Some of the tumor cells that show invasive behaviors undergo polyploidization by endoreplication.
Focusing on these phenomena, we are investigating the conditions underlying invasion hotspots, the triggers for polyploidy through endoreplication, and the functional relationship between polyploidy and tumor invasion. We are also studying whether similar phenomena can be observed in other experimental models (mice, mammalian cultured cells) and even in actual human pathological tissues.
Polyploid Cancer Cell Models in Drosophila. Wang, Y. and Tamori, Y. Genes (2024), 15(1), 96.
The initial stage of tumorigenesis in Drosophila epithelial tissues. Tamori, Y. Advances in Experimental Medicine and Biology (2019), 1167: 87–103.
Cell Competition and tissue homeostasis through compensatory cellular growth. Tamori, Y. and Fujita, Y. Journal of Japanese Biochemical Society (2019), 9: 147-158. (Japanese).

Invasion Hotspots
Invasion Hotspots
発がんは、がん原遺伝子やがん抑制遺伝子を含めた複数の遺伝子の変異が時間とともに蓄積することにより、元々は正常な細胞だったものが新たな形質を獲得してがん細胞へと進化する確率論的なプロセスとして理解されている。実際、上皮組織中に出現した一種類のがん抑制遺伝子に変異を持つ細胞が腫瘍形成に至ることはほとんどないが、その変異細胞にさらに別のがん原性変異が導入されると、その多重変異細胞は過増殖を起こして他臓器への浸潤を始める。このような生体組織内での発がん初期の現象は、ショウジョウバエのようなモデル生物を用いた遺伝学実験により明確に示されてきた。
一方、これまでの私達の研究において、このような強いがん原性を持つとされる多重変異細胞であっても、その多くは上皮層から管腔側へ向かって増殖し良性腫瘍を形成すること、加えて、一種類だけのがん原性変異であっても、上皮組織内に点在する特定のスポットに出現した変異細胞は基底膜を破り間質へ出て浸潤を始めることを発見した。この発見は発がんプロセスにおいて、細胞内の遺伝子変異だけではなく細胞の周囲の微小環境にも決定的な役割があることを示唆している。
この我々が「浸潤ホットスポット」と呼ぶ場所では、もともとの細胞配置や細胞極性に少しの乱れが生じており、この組織構造的な歪みががん細胞が間質へ侵入する際の起点になっているという仮説を持った。現在、この浸潤ホットスポットの形態学的・構造力学的・遺伝学的特徴を徹底的に分析し、このスポットを特徴付ける因子を同定するための研究を行っている。また、そもそもこの浸潤ホットスポットの構造は何のためにあるものなのか、そしてがん細胞はなぜこの浸潤ホットスポットから浸潤を始めるのかについて研究を行っている。
Carcinogenesis is understood as a stochastic process in which mutations in multiple genes, including oncogenes and tumor suppressor genes, accumulate over time, causing what was originally a normal cell to acquire new characteristics and evolve into a cancer cell. In fact, cells mutant for one type of tumor suppressor gene that appear in epithelial tissues rarely lead to tumor formation, but when another oncogenic mutation is introduced into the mutant cells, the multiple mutant cells begin to overproliferate and invade other tissues. Such early events of carcinogenesis in vivo have been demonstrated by genetic experiments using model organisms such as Drosophila melanogaster.
Our previous studies, however, have shown that most of the multiple mutant cells, even those with strong oncogenic properties, proliferate in the epithelial layer toward the luminal side and form benign tumors. At the same time, the multiple mutant cells break the basement membrane and invade the stroma only when they appear at specific locations in the epithelial tissue. In addition, even with only one type of oncogenic mutation, mutant cells that emerged in the specific locations showed similar invasive phenotypes. This finding suggests that not only genetic mutations but also the tissue-intrinsic local microenvironment play a decisive role in the carcinogenic process.
We found that, in these tumor invasion niches that we dubbed "invasion hotspots," there is a slight distortion in the intrinsic cellular arrangement and polarity. This finding led us to hypothesize that this structural distortion makes the spots vulnerable to tumorigenic stimuli and that these spots tend to be the starting point for tumor invasion. We are currently conducting a thorough analysis of the morphological, structural-dynamic, and genetic characteristics of the invasion hotspots to identify the factors that characterize the spots. We are also studying the role of this structure in development and the reason why cancer cells start their invasion from the spots.
Tumor-Cell Invasion Initiates at Invasion Hotspots, an Epithelial Tissue-Intrinsic Microenvironment. Kobayashi, R., Takishima H., Deng, S., Fujita, Y. and Tamori, Y. bioRχiv. 2021.09.28.462102
Tumor hotspots: Tissue-intrinsic oncogenic niche. Kobayashi, R. and Tamori, Y. The Cell (Saibo), “Angiogenesis and immunity in tumor development” (Japanese).
Tissue-intrinsic tumor hotspots: terroir for tumorigenesis. Tamori, Y. and Deng, W.-M. Trends in Cancer (2017), 3: 259–268.
Epithelial tumors originate in tumor hotspots, a tissue-intrinsic microenvironment. Tamori, Y., Suzuki, E. and Deng, W.-M. PLoS Biology (2016), 14(9): e1002537.

Polyploid cancer Cells
Polyploid cancer Cells
悪性腫瘍組織は、遺伝学的背景が異なる様々な変異細胞が混じり合った不均一性の高い状況にあり、この異質な細胞間での相互作用や競合は、がんの進展に重要な関わりを持っていることを示す報告も多い。この多様ながん細胞集団の中に、ゲノム倍数性が通常の2倍体から逸脱して4倍体以上になった多倍体化がん細胞(polyploid cancer cells)と呼ばれるサイズの大きな細胞がしばしば観察されることは古くから知られている。
多倍体化した細胞は放射線や細胞分裂阻害剤など、様々なストレスに対して強い耐性を持っており、多倍体細胞の分裂は不等分裂となりがちで多様な異数体(aneuploid)の娘細胞を生み出すことが報告されているため、がん細胞進化の過程で重要な役割を担っていると考えられるが、これまでに適切な実験モデルがなかったためその本態についてはよく分かっていない。
これまでに、ショウジョウバエ、マウス、哺乳類培養細胞を用いた私達の研究において、ある種の腫瘍細胞は一定の環境条件が揃った場合に、通常の有糸分裂を止め、核内倍加周期へ移行することにより多倍体化することを発見した。この腫瘍細胞の多倍体化は、細胞分裂時の偶発的なエラーによるものではなく、核内倍加周期によって生じるものであることから、生体組織内での条件が揃った場合に必然的に起こる現象であると考えている。さらにこの多倍体化巨細胞になったものの中から浸潤性の細胞が出現することから、多倍体化巨細胞を起点としたゲノム倍数性の変動と悪性形質の進化について研究を進めている。
Malignant tumor tissues contain a highly heterogeneous mixture of various mutant cells with different genetic backgrounds, and many reports indicate that interactions and competition among these heterogeneous cells have an important role in cancer progression. It is known that, within this diverse cancer cell population, polyploid cancer cells in which ploidy increases from normal diploidy to tetraploidy or more are often observed.
Polyploid cells are highly resistant to various stresses such as radiation and mitotic inhibitors, and it has been shown that the mitosis of polyploid cells tends to produce a variety of aneuploid daughter cells, which may play an important role in the process of cancer cell evolution. However, the nature of this process is not well understood due to the lack of appropriate experimental models.
In our studies using Drosophila, mouse, and mammalian cultured cells, we have found that under certain environmental conditions, some types of oncogenic mutant cells stop normal mitosis and become polyploid by entering an endoreplication cycle. These data suggest that this polyploidization of tumor cells is not caused by an accidental error during cell division but by the mitotic-to-endocycle transition and that this phenomenon inevitably occurs when conditions are met in a local tissue environment. In our tumor models, furthermore, we found that invasive cells emerge from these polyploid cells, leading us to study the ploidy dynamics in carcinogenesis and the evolution of malignant traits starting from the polyploid cells.
Polyploid Cancer Cell Models in Drosophila. Wang, Y. and Tamori, Y. Genes (2024), 15(1), 96.

Compensatory cellular hypertrophy
Compensatory cellular hypertrophy
ショウジョウバエでは、様々な組織でゲノムの多倍体化(polyploidization)が生じることはよく知られており、唾液腺の多糸染色体や卵の濾胞細胞などで見られる核内倍加(endoreplication)による多倍体化は、古くから染色体研究や細胞周期研究のモデルとして使われてきた。このような多倍体化は、各々の組織で特定のタンパク質を大量生産するためにプログラムされたものであり、ヒトでも胎盤の栄養膜細胞(trophoblast)、肝細胞(hepatocyte)、心筋細胞(cardiomyocyte)などで見ることができる。また、多倍体のような倍数性が通常の2倍体から逸脱した細胞は、老化やがん化した組織中でもしばしば観察されることが知られているが、これらは遺伝子の変異や細胞分裂の異常などによって引き起こされる、いわば偶発的なものであると考えられる。
私達はこれまでの研究で、細胞分裂が停止した組織での損傷修復において、細胞の損失があった場所の周辺細胞が、有糸分裂ではなく多倍体化による細胞肥大によって補填と修復を行う「補償的細胞肥大」(compensatory cellular hypertrophy, CCH)という現象を発見した。さらに、ショウジョウバエの卵濾胞上皮組織を用いた実験から、この補償的細胞肥大では、組織損傷に伴って周辺細胞に生じる物理的な伸張ストレスが多倍体化を惹起する一因であることが分かってきた。同様な現象は、ショウジョウバエ以外でも、ヒトを含めた哺乳類や脊椎動物の様々な組織(肝臓、角膜内皮、腎臓尿細管、心外膜など)において相次いで報告されており、CCHは分裂停止後の組織において広く保存されている組織修復システムと考えることができる。
Polyploidization of the genome can be observed as a genetically programmed process in the development of multicellular organisms. In Drosophila, the developmental polyploidization in some tissues such as larval salivary glands or ovarian follicular epithelia has been intensely studied as a research model for chromosomal dynamics and cell cycle regulation for a long time. Such polyploidization is programmed to produce large amounts of specific proteins in each tissue and can be seen also in human cells such as placental trophoblasts, hepatocytes, and cardiomyocytes. Although polyploid cells are often observed in aged or cancerous tissues, these polyploidizations are thought to be accidental, caused by gene mutations, or abnormalities in cell division.
Our previous studies have discovered a phenomenon called "compensatory cellular hypertrophy (CCH)" by which an abrupt cell death and a local cell loss in postmitotic tissues are compensated through polyploidization-induced cellular hypertrophic growth without cell division. Furthermore, our genetic experiments using Drosophila follicular epithelia revealed that, in the CCH, physical stretching stress induced by loss of local tissue volume triggers polyploidization of cells in the surrounding area. Similar phenomena have been successively reported not only in other tissues of Drosophila but also in various tissues of vertebrates including human (liver, corneal endothelium, renal tubules, epicardium, etc.). Therefore, CCH can be considered a widely conserved tissue repair system in postmitotic tissues.
Mechanotransduction-mediated epithelial tissue repair through cell competition. Nara, S. and Tamori, Y. Journal of Clinical and Experimental Medicine (Igaku No Ayumi) (2020), 274: 434–441. (Japanese).
Growth of winner cells in cell competition - replacement and compensation. Tamori, Y. Journal of Clinical and Experimental Medicine (Igaku No Ayumi) (2016), Vol. 29, No. 9 (Japanese).
Compensatory tissue repair in cell competition. Tamori, Y. Seitai No Kagaku (2016), Vol. 67, No. 2 (Japanese).
Compensatory cellular hypertrophy: the other strategy for tissue homeostasis. Tamori, Y. and Deng, W.-M. Trends in Cell Biology (2014), 24, 230–237.
Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Tamori, Y. and Deng, W.-M. Developmental Cell (2013), 25, 350–363.